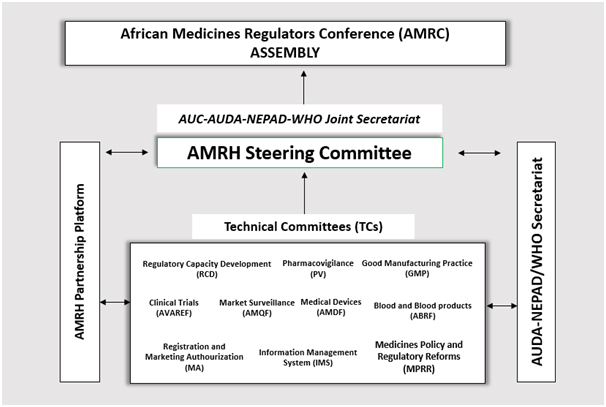
AMRH Governance Structure
The African Medicines Regulatory Initiative (AMRH) was established in 2009 as part of the Pharmaceutical Manufacturing Plan for Africa (PMPA) to provide an enabling environment for facilitating access to good quality, safe and efficacious medical products and health technologies. The initiative which works through regional economic communities (RECs), has established expert working groups and/or technical working groups and steering committees at regional levels supported technically and strategically by the AMRH Technical Committees and a Steering Committee, at continental level. The African Medicines Regulators Conference (AMRC) serves as the AMRH Assembly and serves as a platform for sharing best practices on regulatory matters and a mechanism for generating technical information to guide AU decision making processes’.
As part of alignment of regulatory systems strengthening (RSS), harmonization efforts and networks across the continent, the AMRH has ten technical committees. They include the African Medicines Quality Forum (AMQF) on quality assurance and post marketing surveillance; the African Medical Devices Forum (AMDF); the African Vaccines and the African Vaccines regulatory Forum (AVAREF) for clinical trials and ethics oversight. Pharmacovigilance (PV); the African Blood Regulators Forum (ABRF); Medicines Policy and Regulatory Reforms (MPRR); Regulatory Capacity Development (RCD) and Good Manufacturing Practice (GMP); Registration and Marketing Authorisation (MA) and Information Management System (IMS). The AMRH partnership Platform provides support to the Technical Committees. African Union Development Agency-New Partnership for Africa’s Development (AUDA-NEPAD) and the World Health Organization (WHO) serve as Joint Secretariat for the AMRH Initiative; together with the African Union Commission.
The African Medicines Regulatory Harmonization Governance Framework